REPORT: 2021-2023 UMSAEP Award (R. den Haan)
To: The Director
University of Missouri South African Education Program (UMSAEP) 213 Hulston Hall
Columbia MO 65211
03 November 2023
Dear Prof. Uphoff,
This serves as report of the activities associated with the award made to me at the University of the Western Cape (UWC) under the University of Missouri South African Education Program for research initiated in 2021.
Summary
The funding was granted for me to initiate a collaborative project with Prof. Caixia Wan (Associate Professor, Chemical and Biomedical Engineering, University of Missouri (MU)) that would develop processes for xylitol production from xylose and pretreated biomass derived sugars. The project background, objectives and outcomes are given in greater detail on subsequent pages. At UWC, I identified an MSc student to take up the project. Ms Amber Maneveldt worked on the project that we entitled “Engineering robust yeast strains for the conversion of xylose derived from lignocellulosic biomass to xylitol”. Ms Maneveldt has been successful in achieving most of our initial objectives and is currently completing her MSc thesis. Examiners have been appointed and the thesis will be sent for examination at the end of November 2023. She also presented her results at the recent conference of the South African Society for Microbiology (SASM2023) held in Stellenbosch, South Africa in September. The poster presented is included in this report. At MU, Dr. Wan lab studied the fermentative production of xylitol from hemicellulose hydrolysate derived from lignocellulosic biomass especially using a wild-type yeast strain Candida tropicalis. Ms. Jiayue Chen successfully completed her MSc thesis on this topic at MU. The study in fermenting real hydrolysates of lignocellulosic biomass would provide baseline data for applying engineering strains with high tolerance to inhibitors in the hydrolysate for more efficient xylitol production.
Initially it was hoped that a visit by me to Missouri would be possible. Early in the collaboration I was weary of international travel given the state of the pandemic, and subsequently we were waiting for the construction of the yeast strains as detailed below, which took much longer than expected. Unfortunately, the xylitol production of our strains was lower than expected - a problem we hope to resolve in future. Given this, and the fact that not all the awarded funds were spent, I hope to still travel to Missouri in future to meet Prof. Wan and discuss future collaborative opportunities.
With best regards,
Prof. Riaan den Haan
Associate Professor
Department of Biotechnology
University of the Western Cape
t. 021-959-2199
e. rdenhaan@uwc.ac.za
Project details:
Conversion of xylose derived from lignocellulosic biomass to xylitol.
Recently there has been increased interest in the development of technologies to produce biochemicals from plant biomass for the valorization of waste material, or to broaden the product range of biorefineries. Xylitol, used as a low-calorie sweetener, is one product that has gained attention. Xylitol is a high-value bulk commodity chemical, with established markets and a market price of US$4 500 per tonne, and the market for xylitol has grown to over US$1 billion p/a. Xylitol is produced in fermentation by yeasts including Candida spp. and recombinant Saccharomyces cerevisiae strains. An aspect we would like to address in this collaborative research is the production of xylitol from mixtures of pure xylose and xylo-oligosaccharides, derived from pretreated lignocellulosic biomass. Pretreatment inevitably releases phenolics, furans and organic acids that inhibit yeast performance. Recent reports showed an emerging interest in the application of natural yeast isolates over lab yeast strains or those used in first generation ethanol production as some isolates proved more resistant to inhibitors and may have greater capacity to secrete heterologous enzymes. Such yeasts could provide a superior starting point for engineering host strains for xylitol production. Here, we engineered previously isolated, robust strains of S. cerevisiae to produce xylitol from xylose and xylo-oligosaccharide mixtures. The strain development was actioned by a University of the Western Cape researcher under supervision of Prof. Riaan den Haan. In the collaboration, the pretreatment and process development aspects were supervised by Prof. Caixia Wan from the University of Missouri.
Strain development (UWC)
Aim:
Develop S. cerevisiae strains previously shown to be resistant to pretreatment derived inhibitors for the conversion of xylose and xylo-oligosaccharides to xylitol.
|
Objective: |
Outcome: |
|---|---|
|
Express the xylose reductase encoding genes of Scheffersomyces stipitis and Candida sp. in three robust S. cerevisiae natural isolate strains to establish conversion of xylose to xylitol. A CRISPR-Cas9 based method for stable integration of the genes will be used. |
Only the XR encoding gene of S. stipitis was expressed in our S. cerevisiae strains but all such strains were successfully produced. Xylitol production data show that XR was not produced at the levels we had hoped. Going forward, this activity must be improved in our strains. |
|
Confirm activity of the heterologous xylose reductases in recombinant yeasts in inhibitory fermentative conditions |
Activity was shown to be lower than desired (see previous comment). Expression of Candida XR encoding genes and the endogenous GRE3 will be pursued. |
|
Express β-xylosidase encoding genes in the strains created in objective 1 to allow conversion of xylo-oligosaccharides to xylitol. |
The expression of the β-xylosidase was achieved and an additional β-xylanase encoding gene was also added. High level expression of both was achieved using a CRISPR-Cas9 based method. |
|
Confirm the activities of the heterologous enzymes produced in yeast and test the production of xylitol from xylose and commercial xylo-oligosaccharides at shake flask level. |
All heterologous activities were confirmed and xylitol production from xylose, xylo-oligosaccharides and polymeric xylan was demonstrated, albeit at relatively low levels. |
Process development (MU)
Aim:
Develop a cost-effective process for conversion of xylose and xylo-oligosaccharides directly hydrolyzed from lignocellulosic biomass to xylitol using our engineering yeast strains.
|
Objective: |
Outcome: |
|---|---|
|
Develop the pretreatment processes for producing xylose-rich hydrolysates |
When the real lignocellulosic hydrolysate was used for fermentation, dilute acid pretreatment was effective for releasing fermentable xylose, and deacetylation was used for removing major inhibitors, especially acetic acid, which was the most significant factor to extend lag phase and postpone xylitol production. The maximum xylitol yield was 50% in deacetylated hydrolysate in part due to unbalanced xylose to glucose ratio. Moreover, glucose had the positive effects on inhibitors consumption, and it was indispensable for production of byproduct ethanol. Interestingly, we found that deacetylation was beneficial to accelerate cell growth and xylitol production via removing major inhibitors especially acetic acid. On the other hand, inhibitors had the effects on retarding cell growth and xylitol production but did not affect xylitol yield significantly. |
|
Produce xylitol from xylose-rich biomass hydrolysates by engineered yeast strains |
For xylitol production from lignocellulosic biomass, we first studied the effects of inhibitory compounds typically present in the hydrolysate and ratio of fermentable sugars on a wild-type xylitol-producing yeast strain Candida tropicalis (NRRL-Y607). Both pure sugars and corn cob hydrolysates were used and compared for xylitol production by the strain. The strain’s performance in the production of xylitol and by-product ethanol was investigated. The best results of pure sugar fermentation were obtained with xylose as the majority (i.e., 75%) in the mixture of xylose and glucose. Under this optimal condition, the maximum xylitol yield was 80%. In addition, oxygen was found to be indispensable for cell growth. Higher inoculum size could make xylose consumption more efficiently. Additional glucose was beneficial to cell growth and xylitol production. We anticipate that using engineered yeast strain with high XR expression level would be able to efficiently use non-deacetylated hydrolysate or better convert deacetylated hydrolysates. Further efforts are needed to improve strain’s activities significant before it can be tested for real lignocellulosic hydrolysate. Nevertheless, the characteristic data of hydrolysates and obtained insights into the influence of inhibitors and composition of fermentable sugars on model strain’s activities for xylitol production would provide guidance in fermentation process optimization and further strain improvement. |
Project outputs (UWC):
-
MSc Thesis: A. Maneveldt. “Engineering robust yeast strains for the conversion of xylose derived from lignocellulosic biomass to xylitol” Year of first registration: 2021. Thesis to be sent for examination in November 2023, for graduation in 2024.
-
Poster presentation at SASM2023 conference. Metabolically engineering naturally robust Saccharomyces cerevisiae strains for the conversion of xylose, derived from lignocellulosic biomass, to xylitol. A. Maneveldt &
R. den Haan. Stellenbosch, South Africa, 17-20 September, 2023. (poster included below).
Project outputs (MU):
1. MSc Thesis: Jianyue Chen. “Fermentative production of Xylitol from Hemicellulose Hydrolysate by Candida tropicalis”, University of Missouri, graduated in July 2021.
Metabolically engineering naturally robust Saccharomyces cerevisiae strains for the conversion of xylose, derived from lignocellulosic biomass, to xylitol
A. J. Maneveldt and R. den Haan
Department of Biotechnology, University of the Western Cape, South Africa
Introduction
-
The sustainability of the cellulosic ethanol industry is dependent on adding value through co-products marketed at high prices, thereby increasing the viability of the second-generation (2G) biofuel production 1,2.
-
One such value-added product is xylitol, produced from the lignocellulosic sugar xylose 3.
-
Xylitol has diverse applications across a range of industries, which has driven its market growth and rendered it one of the top ten value-added biochemicals 4-6.
-
Currently, large-scale production is achieved by the chemical reduction of xylose which results in drawbacks including costly production 3, 7-9.
-
Since global xylitol demand is increasing, particular attention has been paid to the biotechnological route where microorganisms reduce xylose to xylitol by the enzyme xylose reductase7, 10.
-
Naturally robust micro-organisms are suggested to be a superior starting point for engineering host strains for xylitol production 11,12.
-
In this study we set out to engineer a xylitol production route in three natural Saccharomyces cerevisiae
Results
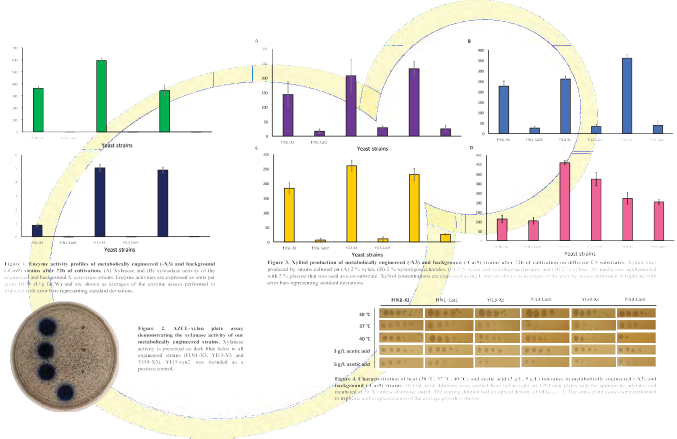
Methodology
-
The genes encoding the enzymes required for xylitol production from hemicellulosic hydrolysate (Trichoderma reesei endo-β-xylanase, Pyrenophora tritici-repentis β-xylosidase, and Scheffersomyces stipitis xylose reductase) were successively incorporated into the hosts’ delta sequences via CRISPR-Cas9 or conventional transformation and were constitutively expressed to convert different C5-sugar sources into xylitol.
-
Heterologous xylanase and xylosidase activities were assayed using previously published methodology 13,14.
-
Xylitol production was quantified using the ᴅ-Xylitol Assay Kit (Megazyme).
-
Tolerance of the naturally robust strains were qualified under appropriate conditions in stress plate tests.
Conclusion
-
Multi-copy integration proved successful, creating transformed strains with xylanase and xylosidase activities significantly higher than previous reported studies 14.
-
When grown on xylan, xylo-oligosaccharides, or both, the engineered strains produced xylitol titres significantly greater than that of the background strains.
-
This is extremely valuable for the industry as it indicates that more crude, economic substrates are suitable for xylitol production instead of costly, pure xylose.
-
When grown on xylose, the xylitol titres of the engineered and background strains were not significantly different, likely due to the native GRE3 gene in S. cerevisiae.
-
Future development can therefore be based on native GRE3 for xylose reduction.
References
-
Lynd, L. R. (2017) ‘The grand challenge of cellulosic biofuels’ Nature Biotechnology: 35 (10): 912-915.
-
Balan, V. (2014) ‘Current Challenges into Commercially Producing Biofuels from Lignocellulosic Biomass’ ISRN Biotechnology: 1-31.
-
Rao, L. V., Goli, J. K., Gentela, J. and Koti, S. (2016) ‘Bioconversion of lignocellulosic biomass to xylitol: An overview’ Bioresource Technology: 213: 299-310.
-
Ahuja, V., Macho, M., Ewe, D., Singh, M., Saha, S. and Saurav, K. (2020) ‘Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism’ Foods: 9(11): 1592.
-
Budzianowski, W. M. (2016) ‘High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries’ Renewable and Sustainable Energy Reviews: 70(2): 793-804.
-
Hernández-Pérez, A. F., de Arruda, P. V., Sene, L., da Silva, S. S., Chandel, A. K. and Felipe, M. G. A. (2019) ‘Xylitol bioproduction: state-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries’ Critical Reviews in Biotechnology: 39(7):924-943.
-
de Albuquerque, T. L., Da Silva, I. J., De MacEdo, G. R. and Rocha, M. V. P. (2014) ‘Biotechnological production of xylitol from lignocellulosic wastes: A review’ Process Biochemistry: 49(11): 1779–1789.
-
Takkellapti, S., Li, T. and Gonzalez, M. A. (2018) ‘An Overview of Biorefinery Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery’ Clean Technologies and Environmental Policy: 20(7): 1615-1630.
-
Ur-Rehman, S., Mushtaq, Z., Zahoor, T., Jamil, A. and Murtaza, M. A. (2015) ‘Xylitol: A Review on Bioproduction, Application, Health Benefits, and Related Safety Issues’ Critical Reviews in Food Science and Nutrition: 55(11): 1514-1528.
-
Ishizaki, H. and Hasumi, K. (2014) ‘Chapter 10 – Ethanol Production from Biomass’, in Tojo, S. and Hirasawa, T. (ed.) Research Approaches to Sustainable Biomass Systems. Tokyo: Academic Press, pp.243-258.
-
Davison, S. A., den Haan, R. and van Zyl, W. H. (2016) ‘Heterologous expression of cellulase genes in natural Saccharomyces cerevisiae strains’ Applied Microbiology and Biotechnology: 100(18): 8241-8254.
-
Jansen, M. L. A., Bracher, J. M., Papapetridis, I., Verhoeven, M. D., de Bruijn, H., de Waal, P. P., van Maris, A. J. A., Klaasen, P. and Pronk, J. T. (2017) ‘Saccharomyces cerevisiae strains for second-generation ethanol production: from academic exploration to industrial implementation’ FEMS Yeast Research: 17(5): fox044.
-
Bailey, M. J., Biely, P. and Poutanen, K. (1992) ‘Interlaboratory testing of methods for assay of xylanase activity’ Journal of Biotechnology: 23: 257-270.
-
Kruger, F. and den Haan, R. (2022) ‘Surface tethered xylosidase activity improved xylan conversion in engineered strains of Saccharomyces cerevisiae’ Journal of Chemical Technology and Biotechnology: 97(5): 1099-1111.
Acknowledgements:
We would like to thank the National Research Foundation for the funds they provided during the first two years of this research.
Reviewed 2025-11-03